
G A chemist prepares a solution of barium acetate by measuring out 107 umol of barium acetate into a ml 200 volumetric flask and filling the flask to the mark with water. Calculate the concentration in of the chemist's barium acetate solution. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1
You know the right answer?
G A chemist prepares a solution of barium acetate by measuring out 107 umol of barium acetate into a...
Questions

Mathematics, 30.10.2019 19:31



Social Studies, 30.10.2019 19:31

Geography, 30.10.2019 19:31

World Languages, 30.10.2019 19:31

Mathematics, 30.10.2019 19:31

English, 30.10.2019 19:31


Geography, 30.10.2019 19:31


Social Studies, 30.10.2019 19:31





Mathematics, 30.10.2019 19:31



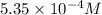
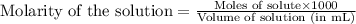
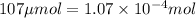 (Conversion factor:
(Conversion factor: 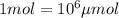 )
)



