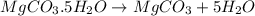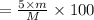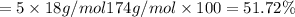You are in space and running out of water. You do have a great deal of magnesium carbonate pentahydrate. It is possible to extract the water from this. Determine the percent of water by mass in the hydrate magnesium carbonate pentahydrate (MgCO_3 middot 5H_2O). If your answer is 17%, enter 17, do not put in the percent sign. As an introduction to his class, Severus Snape teaches the first years at Hogwarts about hydrates. He begins with equations representing various dehydrations. Which of the following equations properly represents the dehydration of magnesium carbonate pentahydrate (MgCO_3 middot 5H_2O)?
MgCO_3 middot H_2O MgCO_3 + H_2O MgCO_3 middot 5H_2O
MgCO_3 + 5H_2O MgCO_3 middot 5H_2O MgCO_3 + H_2O
MgCO_3 middot 5H_2O MgCO_3 + 5H_2O

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

You know the right answer?
You are in space and running out of water. You do have a great deal of magnesium carbonate pentahydr...
Questions



Mathematics, 17.06.2021 18:20



Mathematics, 17.06.2021 18:20


Mathematics, 17.06.2021 18:20

Computers and Technology, 17.06.2021 18:20







Computers and Technology, 17.06.2021 18:20

Health, 17.06.2021 18:20



Mathematics, 17.06.2021 18:20