Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2

Chemistry, 23.06.2019 14:30
Recognizing the properties of water water has a "bent" geometry. which explanation does not explain why? o water's oxygen has unbonded electron pairs that repel each other. water can form hydrogen bonds. electrons are evenly distributed in the water molecule. do ne
Answers: 3
You know the right answer?
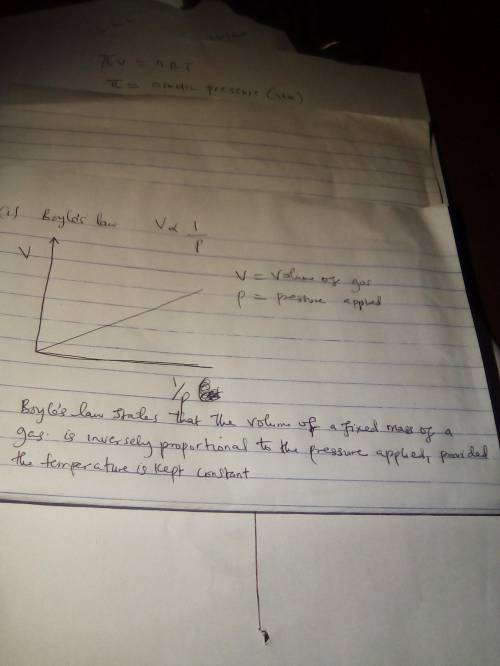
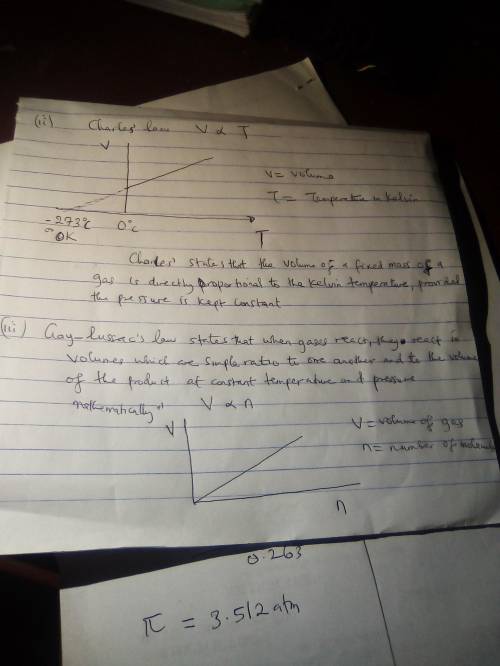
Draw graphs representing Boyle’s law, Charles’s law, and Gay-Lussac’s law for a fixed amount of gas....
Questions



Computers and Technology, 27.11.2019 23:31







History, 27.11.2019 23:31







Mathematics, 27.11.2019 23:31

Mathematics, 27.11.2019 23:31


Mathematics, 27.11.2019 23:31