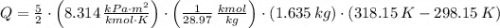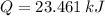Chemistry, 06.03.2020 23:46 reggiegilbert1995
A tank of 0.1m3 volume contains air at 25∘C and 101.33 kPa. The tank is connected to a compressed-air line which supplies air at the constant conditions of 45∘C and 1500 kPa. A valve in the line is cracked so that air flows slowly into the tank until the pressure equals the line pressure. If the process occurs slowly enough that the temperature in the tank remains at 25∘C, how much heat is lost from the tank? Assume air to be an ideal gas for which CP=(7/2)R and CV=(5/2)R.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
A tank of 0.1m3 volume contains air at 25∘C and 101.33 kPa. The tank is connected to a compressed-ai...
Questions

English, 13.01.2021 17:20


Biology, 13.01.2021 17:20



Chemistry, 13.01.2021 17:20

History, 13.01.2021 17:20


Mathematics, 13.01.2021 17:20

Mathematics, 13.01.2021 17:20



Arts, 13.01.2021 17:20

Mathematics, 13.01.2021 17:20



English, 13.01.2021 17:20



Engineering, 13.01.2021 17:20

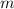 ), in kilograms, within the tank can be found by using the following form:
), in kilograms, within the tank can be found by using the following form: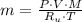 (1)
(1)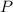 - Pressure, in kilopascals.
- Pressure, in kilopascals. 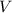 - Volume, in cubic meters.
- Volume, in cubic meters.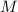 - Molar mass, in kilomoles per kilogram.
- Molar mass, in kilomoles per kilogram.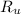 - Ideal gas constant, in kilopascal-cubic meters per kilomole-Kelvin
- Ideal gas constant, in kilopascal-cubic meters per kilomole-Kelvin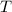 - Temperature, in Kelvin.
- Temperature, in Kelvin.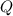 ) occur in a isochoric process, that is, a process at constant volume, it is caused by the heat released by the air flowing to the tank. The formula is represented by the following application of the definition of sensible heat:
) occur in a isochoric process, that is, a process at constant volume, it is caused by the heat released by the air flowing to the tank. The formula is represented by the following application of the definition of sensible heat: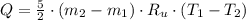 (2)
(2)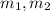 - Initial and final masses of the air within the tank, in kilograms.
- Initial and final masses of the air within the tank, in kilograms.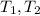 - Initial and final temperatures of the air inflow, in Kelvin.
- Initial and final temperatures of the air inflow, in Kelvin.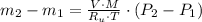 (3)
(3)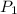 and
and 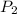 are the initial and final pressures of the air inside the tank, in kilopascals.
are the initial and final pressures of the air inside the tank, in kilopascals. 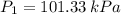 ,
, 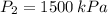 ,
, 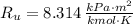 ,
, 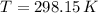 ,
, 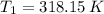 ,
, 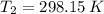 ,
,  and
and 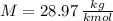 , then the heat losses are:
, then the heat losses are:![m_{2} - m_{1} = \left[\frac{(0.1\,m^{3})\cdot \left(28.97\,\frac{kg}{kmol} \right)}{\left(8.314\,\frac{kPa\cdot m^{2}}{kmol\cdot K} \right)\cdot (298.15\,K)} \right]\cdot (1500\,kPa-101.325\,kPa)](/tpl/images/0536/5617/f9891.png)