Chemistry, 06.03.2020 21:26 tommyaberman
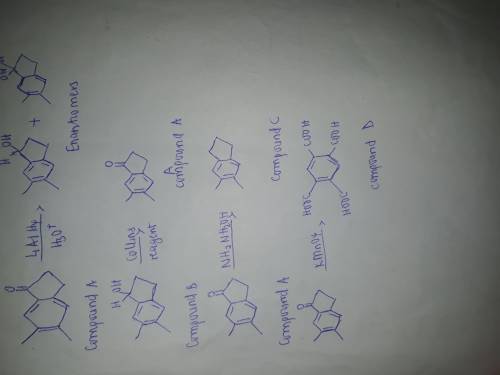
Compound A, C11H12O, which have a negative Tollens test, was treated with LiAlH4, followed by dilute acid, to give compound B, which could be resolved into enantiomers. When optically active B was treated with CrO3 in pyridine, an optically inactive sample of A was obtained. Heating A with hydrazine in base gave hydrocarbon C, which, when heated with alkaline KMnO4, gave carboxylic acid D. Identify compounds A, B, and C.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
You know the right answer?
Compound A, C11H12O, which have a negative Tollens test, was treated with LiAlH4, followed by dilute...
Questions

Geography, 18.12.2020 01:00

Biology, 18.12.2020 01:00

History, 18.12.2020 01:00



Mathematics, 18.12.2020 01:00


Mathematics, 18.12.2020 01:00

Chemistry, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00



English, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00



Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00