
A 0.10 mol sample of each of the four species in the reaction represented above is injected into a rigid, previously evacuated 1.0 L container. Which of the following species will have the highest concentration when the system reaches equilibrium?
a. H2S(g)
b. CH4(g)
c. CS2(g)
d. H2(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
A 0.10 mol sample of each of the four species in the reaction represented above is injected into a r...
Questions

Social Studies, 30.08.2021 16:20




Mathematics, 30.08.2021 16:30

Mathematics, 30.08.2021 16:30

Mathematics, 30.08.2021 16:30

English, 30.08.2021 16:30

English, 30.08.2021 16:30



Mathematics, 30.08.2021 16:30




Engineering, 30.08.2021 16:30

Health, 30.08.2021 16:30

English, 30.08.2021 16:30


Mathematics, 30.08.2021 16:30

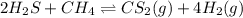
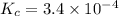
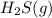
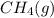
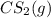
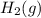
![[H_2S]=\frac{0.10 mol}{1.0 L}=0.10 M](/tpl/images/0535/2301/dae4c.png)
![[CH_4]=\frac{0.10 mol}{1.0 L}=0.10 M](/tpl/images/0535/2301/980f6.png)
![[CS_2]=\frac{0.10 mol}{1.0 L}=0.10 M](/tpl/images/0535/2301/48b85.png)
![[H_2]=\frac{0.10 mol}{1.0 L}=0.10 M](/tpl/images/0535/2301/1e4fd.png)
![Q_c=\frac{[CS_2][H_2]^4}{[H_2S]^2[CH_4]}](/tpl/images/0535/2301/ae8a1.png)
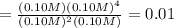
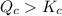 (reaction will go backward)
(reaction will go backward)![K_c=\frac{[CS_2][H_2]^4}{[H_2S]^2[CH_4]}](/tpl/images/0535/2301/6e60b.png)
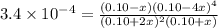
![=[H_2S]=(0.10+2x) M=(0.10+2\times 0.099) M=0.298 M](/tpl/images/0535/2301/fb008.png)


