
Chemistry, 06.03.2020 02:44 serehnatyras0808
If 1 10 liter sample of O2 gas at 300 kelvin and 0.5 atmosphere of pressure contains 5.0 x 10^22 molecules how many molecules would a 10 liter sample of H2 have if it was at the same temperature and pressure as the oxygen remember that hydrogens are 1/16th the size of oxygen

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
If 1 10 liter sample of O2 gas at 300 kelvin and 0.5 atmosphere of pressure contains 5.0 x 10^22 mol...
Questions

History, 02.11.2020 08:00

Mathematics, 02.11.2020 08:00


Medicine, 02.11.2020 08:00


Mathematics, 02.11.2020 08:00


Advanced Placement (AP), 02.11.2020 08:00





Mathematics, 02.11.2020 08:00


Mathematics, 02.11.2020 08:10


Social Studies, 02.11.2020 08:10


Mathematics, 02.11.2020 08:10

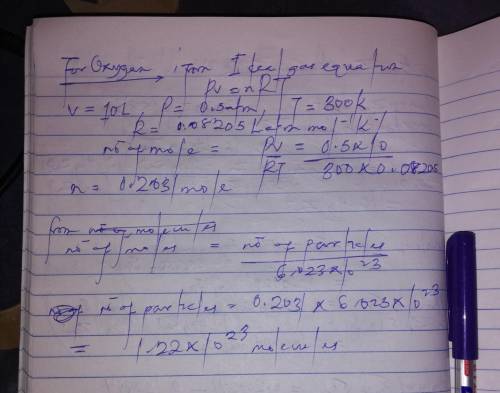
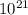
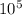 pa
pa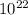 molecules
molecules J/K.
J/K.

