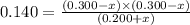Consider mixture C, which will cause the net reaction to proceed in reverse. Concentration (M)initial:change:equilibrium:[XY]0 .200+x0.200+x←net⇌[X]0.300−x0.300−x +[Y]0.300−x0.300−x The change in concentration, x, is positive for the reactants because they are produced and negative for the products because they are consumed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
Consider mixture C, which will cause the net reaction to proceed in reverse. Concentration (M)initia...
Questions


Mathematics, 25.01.2022 03:50

Social Studies, 25.01.2022 03:50


Business, 25.01.2022 03:50

Mathematics, 25.01.2022 03:50

Biology, 25.01.2022 03:50

Geography, 25.01.2022 03:50

Mathematics, 25.01.2022 03:50



Mathematics, 25.01.2022 03:50


Mathematics, 25.01.2022 03:50


Chemistry, 25.01.2022 03:50

Mathematics, 25.01.2022 03:50


Mathematics, 25.01.2022 04:00

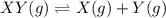
![K_c=\farc{[X][Y]}{[XY]}](/tpl/images/0534/4698/91cd9.png)