

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 08:30
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 2


Chemistry, 23.06.2019 18:50
Was there supercooling? would you expect the water or the sugar solution to have the most supercooling? why?
Answers: 2
You know the right answer?
. The molar heat of vaporization of acetone, C3H6O, is 30.3 kJ/mol at its boiling point. How many ki...
Questions








Social Studies, 10.12.2020 17:20


Mathematics, 10.12.2020 17:20

Biology, 10.12.2020 17:20

Engineering, 10.12.2020 17:20



Mathematics, 10.12.2020 17:20

English, 10.12.2020 17:20



Mathematics, 10.12.2020 17:20

Biology, 10.12.2020 17:20

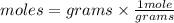
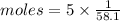
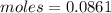
 heat of vapourization
heat of vapourization

