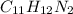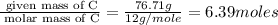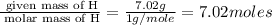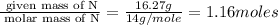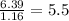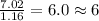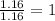Chemistry, 05.03.2020 04:15 angelicar4144
The mass composition of a compound that assists in the coagulation of blood is 76.71% carbon, 7.02% hydrogen, and 16.27% nitrogen. Determine the empirical formula of the compound and report the answer by specifying X, Y & Z in the format below:
C_X H_Y N_Z

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
The mass composition of a compound that assists in the coagulation of blood is 76.71% carbon, 7.02%...
Questions

History, 19.07.2019 10:30






Mathematics, 19.07.2019 10:30

Mathematics, 19.07.2019 10:30

Spanish, 19.07.2019 10:30

History, 19.07.2019 10:30



English, 19.07.2019 10:30

Biology, 19.07.2019 10:30

Chemistry, 19.07.2019 10:30



Mathematics, 19.07.2019 10:30

Computers and Technology, 19.07.2019 10:30