
Chemistry, 05.03.2020 03:40 joThompson
When this system is at equilibrium at a certain temperature PCl5(g) ⇋ PCl3(g) + Cl2(g), the concentrations are found to be [PCl5] = 0.40 M, [PCl3] = [Cl2] = 0.20. If the volume of the container is suddenly halved at the same temperature, what will be the new equilibrium concentration of PCl5?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
You know the right answer?
When this system is at equilibrium at a certain temperature PCl5(g) ⇋ PCl3(g) + Cl2(g), the concentr...
Questions

Social Studies, 15.12.2020 17:10


Business, 15.12.2020 17:10


Social Studies, 15.12.2020 17:10


Mathematics, 15.12.2020 17:10


Mathematics, 15.12.2020 17:10



Mathematics, 15.12.2020 17:10

Mathematics, 15.12.2020 17:10


Social Studies, 15.12.2020 17:10

Mathematics, 15.12.2020 17:10

Mathematics, 15.12.2020 17:10

Mathematics, 15.12.2020 17:10

Mathematics, 15.12.2020 17:10

English, 15.12.2020 17:10

 will be 0.9 M.
will be 0.9 M.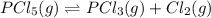
![[PCl_5]=0.40 M](/tpl/images/0534/1154/cb9c6.png)
![[PCl_3]=[Cl_2]=0.20 M](/tpl/images/0534/1154/9bc6a.png)
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0534/1154/73fe0.png)
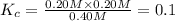

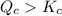
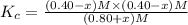
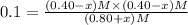
![[PCl_5]=(0.80+x) M=(0.80+0.1) M = 0.90](/tpl/images/0534/1154/dbad2.png)


