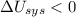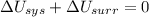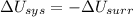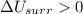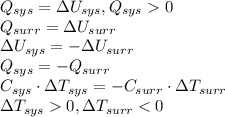Chemistry, 04.03.2020 23:29 isaiahcannon5709
Determine if each of the statements is True or False regarding the First Law of Thermodynamics.
1. If the system loses energy to the surroundings, the surroundings could also lose energy to the system.
2. If the system gains thermal energy from the surroundings, the temperature of the surroundings decreases.
3 If the system gains 25 kJ of energy from the surroundings without doing any work on the surroundings, the surroundings could lose 20 kJ of energy.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1


Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
Determine if each of the statements is True or False regarding the First Law of Thermodynamics.
Questions


Mathematics, 19.04.2021 04:50

Mathematics, 19.04.2021 04:50




Health, 19.04.2021 04:50

Mathematics, 19.04.2021 04:50

Mathematics, 19.04.2021 04:50

Mathematics, 19.04.2021 04:50

English, 19.04.2021 04:50


Mathematics, 19.04.2021 04:50


Mathematics, 19.04.2021 04:50


Mathematics, 19.04.2021 04:50