
A magnesium hydroxide solution is prepared by adding 10.00 g of magnesium hydroxide to a volumetric flask and bringing the final volume to 1.00 L by adding water buffered at a pH of 7.0. What is the concen- tration of magnesium in this solution? (Assume that the temperature is 25◦C and the ionic strength is negligible).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2


Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
A magnesium hydroxide solution is prepared by adding 10.00 g of magnesium hydroxide to a volumetric...
Questions

Mathematics, 20.03.2021 03:00



Mathematics, 20.03.2021 03:00




English, 20.03.2021 03:00

Mathematics, 20.03.2021 03:00


Mathematics, 20.03.2021 03:00

English, 20.03.2021 03:00


Mathematics, 20.03.2021 03:00


Mathematics, 20.03.2021 03:00

Mathematics, 20.03.2021 03:00

Biology, 20.03.2021 03:00


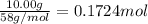
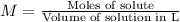
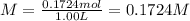
![[Mg^{2+}]=1\times 0.1724 M=0.1724 M](/tpl/images/0533/7652/0d10f.png)



