Chemistry, 04.03.2020 01:53 smithsa10630
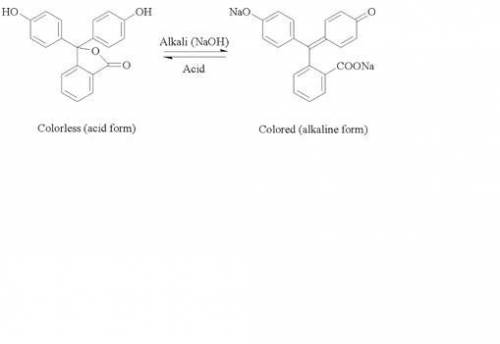
When a strong acid is titrated with a strong base using pheolphthalein as an indicator, the color changes abruptly at the endpoint of the titration and can be switched back and forth by the addition of only one drop of acid or base. The reason for the abruptness of this color change is that:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1


Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1

You know the right answer?
When a strong acid is titrated with a strong base using pheolphthalein as an indicator, the color ch...
Questions

History, 19.03.2021 08:50

Mathematics, 19.03.2021 08:50

Mathematics, 19.03.2021 08:50



Physics, 19.03.2021 08:50

World Languages, 19.03.2021 08:50

Mathematics, 19.03.2021 08:50

Mathematics, 19.03.2021 08:50

Biology, 19.03.2021 08:50

Mathematics, 19.03.2021 08:50


Mathematics, 19.03.2021 08:50

Mathematics, 19.03.2021 08:50




Mathematics, 19.03.2021 08:50

Physics, 19.03.2021 08:50