
Chemistry, 03.03.2020 22:53 tejasheree
In acidic aqueous solution, the purple complex ion Co(NH3)5Br2+ undergoes a slow reaction in which the bromide ion is replaced by a water molecule, yielding the pinkish-orange complex ion : Co(NH3)5(H2O)3+
Co(NH3)5Br2+Purple(aq)+H2O(l)? Co(NH3)5(H2O)3+Pinkish?orange(aq)+B r?(aq...
The reaction is first order in Co(NH3)5Br2+, the rate constant at 25 ?C is 6.3�10?6 s?1, and the initial concentration of Co(NH3)5Br2+ is 0.100 M.
A; What is its molarity after a reaction time of 19.0h ?
B; How many hours are required for 69% of the Co(NH3)5Br2+ to react?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1


Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
In acidic aqueous solution, the purple complex ion Co(NH3)5Br2+ undergoes a slow reaction in which t...
Questions




Mathematics, 15.04.2020 21:05


Mathematics, 15.04.2020 21:05

Mathematics, 15.04.2020 21:05


Mathematics, 15.04.2020 21:05

History, 15.04.2020 21:05

Mathematics, 15.04.2020 21:05



Chemistry, 15.04.2020 21:05

Physics, 15.04.2020 21:05

History, 15.04.2020 21:05

Biology, 15.04.2020 21:05


Mathematics, 15.04.2020 21:05

Mathematics, 15.04.2020 21:05

![[Co(NH_3)5Br]^{2+}](/tpl/images/0532/7758/e70dd.png) will react 69% of its initial concentration.
will react 69% of its initial concentration.![Co(NH_3)_5(H_2O)_3+[Co(NH_3)5Br]^{2+}(Purple)(aq)+H_2O(l)\rightarrow [Co(NH_3)_5(H_2O)]^{3+}(Pinkish-orange)(aq)+Br^-(aq)](/tpl/images/0532/7758/11a87.png)
![[A_o]=0.100 M](/tpl/images/0532/7758/bacc4.png)
![[A]](/tpl/images/0532/7758/6aa06.png)
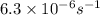
![[A]=[A_o]\times e^{-kt}](/tpl/images/0532/7758/abdec.png)
![[A]=0.100 M\times e^{-6.3\times 10^{-6} s^{-1}\times 19.0\times 3600 s}](/tpl/images/0532/7758/b79ba.png)
![[A]=0.065 M](/tpl/images/0532/7758/56ac3.png)
![[A_o]=x](/tpl/images/0532/7758/aecde.png)
![[A]=(100\%-69\%) x=31\%x=0.31x](/tpl/images/0532/7758/34138.png)
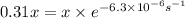
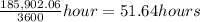 ≈ 52 hours
≈ 52 hours

