
Chemistry, 03.03.2020 17:11 AgarioEdit
Calculate the amount of heat needed to melt 91.5g of solid benzene ( C6H6 ) and bring it to a temperature of 60.6°C . Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1


Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Calculate the amount of heat needed to melt 91.5g of solid benzene ( C6H6 ) and bring it to a temper...
Questions

Mathematics, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

Chemistry, 12.02.2021 14:00



Mathematics, 12.02.2021 14:00




Chemistry, 12.02.2021 14:00



Chemistry, 12.02.2021 14:00

History, 12.02.2021 14:00


English, 12.02.2021 14:00


Mathematics, 12.02.2021 14:00


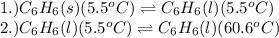
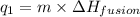
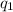 = amount of heat absorbed = ?
= amount of heat absorbed = ?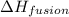 = enthalpy change for fusion = 127.40 J/g
= enthalpy change for fusion = 127.40 J/g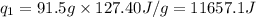
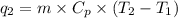
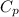 = specific heat capacity of benzene = 1.73 J/g°C
= specific heat capacity of benzene = 1.73 J/g°C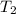 = final temperature = 60.6°C
= final temperature = 60.6°C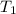 = initial temperature = 5.5°C
= initial temperature = 5.5°C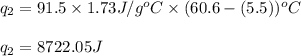
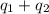
![[11657.1+8722.05]J=20379.2=20.38kJ](/tpl/images/0532/2114/b62c3.png)


