
Chemistry, 03.03.2020 06:05 Frenchfries13
Consider the reaction: N2(g) + 2O2(g)2NO2(g) Using standard absolute entropies at 298K, calculate the entropy change for the system when 1.90 moles of N2(g) react at standard conditions. S°system = J/K Submit Answer

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 10:30
If a 20.0ml test tube measures 15.0cm, what is the length in meters?
Answers: 1
You know the right answer?
Consider the reaction: N2(g) + 2O2(g)2NO2(g) Using standard absolute entropies at 298K, calculate th...
Questions


Mathematics, 18.11.2020 18:50


Mathematics, 18.11.2020 18:50

Mathematics, 18.11.2020 18:50

Mathematics, 18.11.2020 18:50

Business, 18.11.2020 18:50

Mathematics, 18.11.2020 18:50

Mathematics, 18.11.2020 18:50

Mathematics, 18.11.2020 18:50

Biology, 18.11.2020 18:50



Biology, 18.11.2020 18:50



English, 18.11.2020 18:50



Mathematics, 18.11.2020 18:50

 is 191.6 J/mol K,
is 191.6 J/mol K,  = 205 J/mol K, and
= 205 J/mol K, and  is 239.7 J/mol K at 298 K.
is 239.7 J/mol K at 298 K.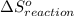 from standard absolute entropies as follows.
from standard absolute entropies as follows.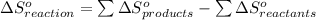
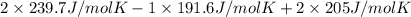
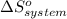 = 1.90 moles of
= 1.90 moles of 


