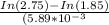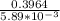Chemistry, 03.03.2020 05:45 Mariela2699
N2O5 decomposes to form NO2 and O2 with first-order kinetics. How long does it take for the N2O5 concentration to decrease from its initial value of 2.75 M to its final value of 1.85 M, if the rate constant, k, equals 5.89 × 10−3?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

You know the right answer?
N2O5 decomposes to form NO2 and O2 with first-order kinetics. How long does it take for the N2O5 con...
Questions

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Biology, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Social Studies, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Physics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

History, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Chemistry, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01