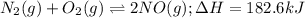Chemistry, 03.03.2020 05:02 emanihackle9
Nitrogen and oxygen react at high temperatures. N2(g) + O2(g) equilibrium reaction arrow 2 NO(g) ΔH = 182.6 kJ (a) What will happen to the concentrations of N2, O2, and NO at equilibrium if more O2 is added?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 23.06.2019 06:30
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3
You know the right answer?
Nitrogen and oxygen react at high temperatures. N2(g) + O2(g) equilibrium reaction arrow 2 NO(g) ΔH...
Questions


Chemistry, 04.09.2019 19:10

History, 04.09.2019 19:10

Physics, 04.09.2019 19:10


History, 04.09.2019 19:10

Physics, 04.09.2019 19:10

Mathematics, 04.09.2019 19:10






Mathematics, 04.09.2019 19:10