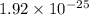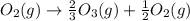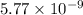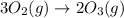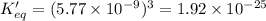Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Given the value of the equilibrium constant (Kc) for the equation (a), calculate the equilibrium con...
Questions

Mathematics, 03.09.2020 04:01


Mathematics, 03.09.2020 04:01

Chemistry, 03.09.2020 04:01

Social Studies, 03.09.2020 04:01



Advanced Placement (AP), 03.09.2020 04:01


History, 03.09.2020 04:01



English, 03.09.2020 04:01






Mathematics, 03.09.2020 04:01