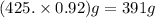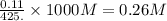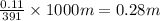A student dissolves 14.g of benzoic acid C7H6O2 in 425.mL of a solvent with a density of 0.92 g/mL. The student notices that the volume of the solvent does not change when the benzoic acid dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1

Chemistry, 23.06.2019 09:00
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2

Chemistry, 23.06.2019 12:20
Amatch has about 21 milligrams of red phosphorus coating the tip. how many atoms of phosphorus is this?
Answers: 1

Chemistry, 23.06.2019 14:30
If energy was included in a chemical reaction, on which side of the equation would it be written for an endothermic reaction?
Answers: 1
You know the right answer?
A student dissolves 14.g of benzoic acid C7H6O2 in 425.mL of a solvent with a density of 0.92 g/mL....
Questions


Computers and Technology, 29.05.2020 17:59



Mathematics, 29.05.2020 17:59

Health, 29.05.2020 17:59




Chemistry, 29.05.2020 17:59






Mathematics, 29.05.2020 17:59



Spanish, 29.05.2020 17:59

History, 29.05.2020 17:59

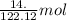 of benzoic acid = 0.11 mol of benzoic acid
of benzoic acid = 0.11 mol of benzoic acid