
Chemistry, 03.03.2020 02:40 RicoCheT89
A solution of hydrochloric acid of unknown concentration was titrated with .21 M NaOH. if a 75 ml sample of the HCl solution required exactly 13ml of the NaOH solution to reach the equivalence point, what was the ph of the HCl solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
A solution of hydrochloric acid of unknown concentration was titrated with .21 M NaOH. if a 75 ml sa...
Questions



Business, 10.01.2020 09:31

History, 10.01.2020 09:31


Mathematics, 10.01.2020 09:31

Mathematics, 10.01.2020 09:31

Mathematics, 10.01.2020 09:31

Mathematics, 10.01.2020 09:31


Social Studies, 10.01.2020 09:31


Mathematics, 10.01.2020 09:31

Business, 10.01.2020 09:31






Chemistry, 10.01.2020 09:31


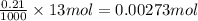
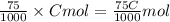
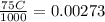
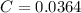
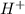
![[H^{+}]=0.0364M](/tpl/images/0531/5477/87d10.png)
![pH=-log[H^{+}]=-log(0.0364)=1.44](/tpl/images/0531/5477/9f9b6.png)


