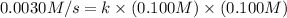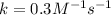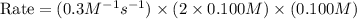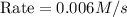Chemistry, 03.03.2020 02:24 batmanmarie2004
When the concentrations of CH 3 Br and NaOH are both 0.100 M, the rate of the reaction is 0.0030 M/s. What is the rate of the reaction if the concentration of CH 3 Br is doubled?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
When the concentrations of CH 3 Br and NaOH are both 0.100 M, the rate of the reaction is 0.0030 M/s...
Questions

Arts, 12.01.2021 20:30

Mathematics, 12.01.2021 20:30

Mathematics, 12.01.2021 20:30





Physics, 12.01.2021 20:30

Mathematics, 12.01.2021 20:30



Social Studies, 12.01.2021 20:30

Mathematics, 12.01.2021 20:30







Social Studies, 12.01.2021 20:30

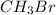 is doubled is, 0.006 M/s
is doubled is, 0.006 M/s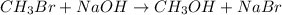
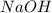 are the reactants.
are the reactants.![\text{Rate}=k[CH_3Br][NaOH]](/tpl/images/0531/5144/64424.png)
![[CH_3Br]](/tpl/images/0531/5144/0572a.png) = concentration of
= concentration of ![[NaOH]](/tpl/images/0531/5144/119b0.png) = concentration of
= concentration of