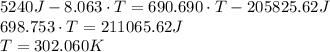A 62.5-g piece of gold at 650. K is dropped into 165 g of H2O (l) at 298 K in an insulated container at 1 bar pressure. Calculate the temperature of the system once equilibrium has been reached. Assume that CP, m for Au and H2O are constant at their values for 298 K throughout the temperature range of interest.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
The wilson chamber is used to study: direction, speed, and distance of radioactivity the intensity of radiation the appearance of individual atoms all of the above
Answers: 1

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
A 62.5-g piece of gold at 650. K is dropped into 165 g of H2O (l) at 298 K in an insulated container...
Questions





Mathematics, 19.12.2019 18:31

Biology, 19.12.2019 18:31

Mathematics, 19.12.2019 18:31


Geography, 19.12.2019 18:31

Biology, 19.12.2019 18:31

History, 19.12.2019 18:31



Mathematics, 19.12.2019 18:31


Biology, 19.12.2019 18:31


Mathematics, 19.12.2019 18:31


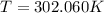
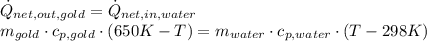
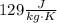 and
and 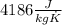 . Last expression is simplified by substituting known variables:
. Last expression is simplified by substituting known variables: