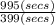An open tubular gas chromatography column is 35.7 m long and has an inner diameter of 0.250 mm. It is coated on the inside wall with a layer of stationary phase that is 1.8 µm (0.0018 mm) thick. Unretained solute, methane, passes through in 6.39 min, whereas toluene has a retention time of 16.35 min. Calculate the retention factor for toluene.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
An open tubular gas chromatography column is 35.7 m long and has an inner diameter of 0.250 mm. It i...
Questions







Mathematics, 14.03.2020 05:08


Mathematics, 14.03.2020 05:08

Mathematics, 14.03.2020 05:08

Chemistry, 14.03.2020 05:08








History, 14.03.2020 05:09

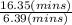 =
=