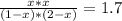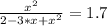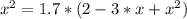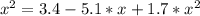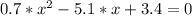Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
The reversible chemical reaction A+B⇌C+D has the following equilibrium constant: Kc=[C][D][A][B]=1.7...
Questions



History, 26.08.2019 15:30

Mathematics, 26.08.2019 15:30




Mathematics, 26.08.2019 15:30


Social Studies, 26.08.2019 15:30

Mathematics, 26.08.2019 15:30






Biology, 26.08.2019 15:30


Mathematics, 26.08.2019 15:30

English, 26.08.2019 15:30

![Kc=\frac{[C]^{c} *[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0531/2647/eda24.png)
![Kc=\frac{[C]*[D]}{[A]*[B]}=1.7](/tpl/images/0531/2647/e4842.png)