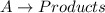Consider a simple reaction in which a reactant AA forms products: A→productsA→products What is the rate law if the reaction is zero order with respect to AA? First order? Second order? For each case, explain how a doubling of the concentration of AA would affect the rate of reaction.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
Consider a simple reaction in which a reactant AA forms products: A→productsA→products What is the r...
Questions


Mathematics, 09.12.2021 21:30


Spanish, 09.12.2021 21:30



Medicine, 09.12.2021 21:30

SAT, 09.12.2021 21:30

Mathematics, 09.12.2021 21:30


Mathematics, 09.12.2021 21:30







Mathematics, 09.12.2021 21:30

Chemistry, 09.12.2021 21:30

![Rate=k[A]^0](/tpl/images/0531/2473/c3515.png)
![Rate=k[A]^1](/tpl/images/0531/2473/044b8.png)
![Rate=k[A]^2](/tpl/images/0531/2473/e6b6e.png)