Chemistry, 02.03.2020 23:58 Dogtes9667
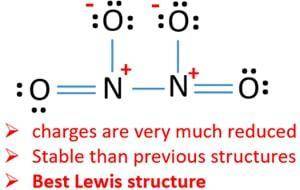
The N−N bond length in N2O is 1.12 Å, slightly longer than a typical N≡N bond, which is 1.10 Å, and the N−O bond length is 1.19 Å, slightly shorter than a typical N=O bond, which is 1.22 Å. Based on these data, which resonance structure best represents N2O?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2
You know the right answer?
The N−N bond length in N2O is 1.12 Å, slightly longer than a typical N≡N bond, which is 1.10 Å, and...
Questions




Mathematics, 05.11.2020 01:00


Mathematics, 05.11.2020 01:00


Mathematics, 05.11.2020 01:00

English, 05.11.2020 01:00

Computers and Technology, 05.11.2020 01:00




Social Studies, 05.11.2020 01:00

World Languages, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00


History, 05.11.2020 01:00

Health, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00