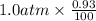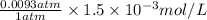Chemistry, 02.03.2020 22:57 brianadee800
Argon makes up 0.93% by volume of air. Calculate its solubility (in mol/L) in water at 20°C and 1.0 atm. The Henry's law constant for Ar under these conditions is 1.5 × 10−3 mol/L·atm.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2


Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2
You know the right answer?
Argon makes up 0.93% by volume of air. Calculate its solubility (in mol/L) in water at 20°C and 1.0...
Questions


Mathematics, 20.05.2021 14:30







Mathematics, 20.05.2021 14:30

Mathematics, 20.05.2021 14:30

Chemistry, 20.05.2021 14:30


Engineering, 20.05.2021 14:30


Physics, 20.05.2021 14:30



Mathematics, 20.05.2021 14:30

Mathematics, 20.05.2021 14:30

Mathematics, 20.05.2021 14:40

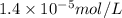
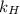 = Henry's law constant of argon =
= Henry's law constant of argon =