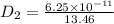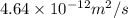Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

You know the right answer?
The activation energy for the diffusion of carbon in chromium is 111,000 J/mol. Calculate the diffus...
Questions

Mathematics, 27.01.2020 19:31

Social Studies, 27.01.2020 19:31

Geography, 27.01.2020 19:31


Spanish, 27.01.2020 19:31

Social Studies, 27.01.2020 19:31




History, 27.01.2020 19:31

History, 27.01.2020 19:31

History, 27.01.2020 19:31

English, 27.01.2020 19:31

Mathematics, 27.01.2020 19:31




Spanish, 27.01.2020 19:31

Mathematics, 27.01.2020 19:31

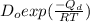
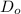 = constant
= constant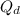 = activation energy
= activation energy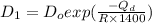
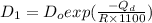
![\frac{D_{1}}{D_{2}} = exp[\frac{-Q_{d}}{1400 R} + \frac{Q}{1100 R}]](/tpl/images/0530/9405/91326.png)
![\frac{6.25 \times 10^{-11}}{D_{2}} = exp [\frac{-Q_{d}}{R}(\frac{-300}{1400 \times 1100})]](/tpl/images/0530/9405/0c339.png)
 = exp (2.6)
= exp (2.6)