
Chemistry, 02.03.2020 22:42 shelbycg02
When 1.00 g of thiamine hydrochloride (also called vitamin B1 hydrochloride) is dissolved in water, then diluted to 10.00 mL, the pH of the resulting solution is 4.50. The formula weight of thiamine hydrochloride is 337.3 g/mol. Calculate Ka.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1


Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
You know the right answer?
When 1.00 g of thiamine hydrochloride (also called vitamin B1 hydrochloride) is dissolved in water,...
Questions





History, 13.11.2019 19:31

Mathematics, 13.11.2019 19:31

Engineering, 13.11.2019 19:31




Spanish, 13.11.2019 19:31

Mathematics, 13.11.2019 19:31



Mathematics, 13.11.2019 19:31


Social Studies, 13.11.2019 19:31

Mathematics, 13.11.2019 19:31


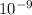
![\frac{[H+] [A-]}{[HA]}](/tpl/images/0530/9044/5a54d.png)
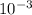
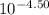
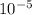
![\frac{[0.0000316] [0.000316]}{0.296}](/tpl/images/0530/9044/484a7.png)


