
Chemistry, 02.03.2020 21:50 youngchapo813p8d9u1
If the pH of a somewhat dilute (i. e. ~0.08 to 0.10 M) aqueous solution of HCl is 1.14, then to a good approximation, what is the concentration of hydrogen ions in this solution? Enter your answer to the nearest hundredth.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3
You know the right answer?
If the pH of a somewhat dilute (i. e. ~0.08 to 0.10 M) aqueous solution of HCl is 1.14, then to a go...
Questions

Mathematics, 19.12.2020 01:00

Chemistry, 19.12.2020 01:00

Mathematics, 19.12.2020 01:00

Mathematics, 19.12.2020 01:00


History, 19.12.2020 01:00

Arts, 19.12.2020 01:00

Mathematics, 19.12.2020 01:00



Mathematics, 19.12.2020 01:00

Mathematics, 19.12.2020 01:00

Mathematics, 19.12.2020 01:00


English, 19.12.2020 01:00

English, 19.12.2020 01:00


Mathematics, 19.12.2020 01:00

Mathematics, 19.12.2020 01:00

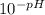 =
= 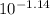 = 0.0724 M= 0.07 M
= 0.0724 M= 0.07 M

