
Chemistry, 02.03.2020 21:03 liljobe8973
C6H5NH3Cl is a chloride salt with an acidic cation. If 46.3 g of C6H5NH3Cl is dissolved in water to make 150 mL of solution, what is the initial molarity of the cation

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
C6H5NH3Cl is a chloride salt with an acidic cation. If 46.3 g of C6H5NH3Cl is dissolved in water to...
Questions

Social Studies, 18.03.2020 03:36



Mathematics, 18.03.2020 03:37

Mathematics, 18.03.2020 03:37

Spanish, 18.03.2020 03:38







Chemistry, 18.03.2020 03:42



Mathematics, 18.03.2020 03:43



Chemistry, 18.03.2020 03:43

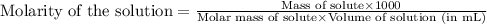
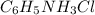 = 46.3 g
= 46.3 g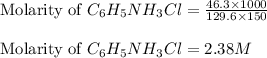
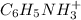 cation and 1 mole of
cation and 1 mole of 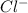 anion
anion

