
Chemistry, 02.03.2020 21:07 jahnoibenjamin
Which of the following solutions would have the highest [H3O+]? Assume that they are all 0.10 M in acid at 25∘C. The acid is followed by its Ka value. C) HNO2, 4.6 × 10-4 B) HCN, 4.9 × 10-10 E) HClO2, 1.1 × 10-2 A) HF, 3.5 × 10-4 D) HCHO2, 1.8 × 10-4

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

You know the right answer?
Which of the following solutions would have the highest [H3O+]? Assume that they are all 0.10 M in a...
Questions

Social Studies, 29.01.2020 01:54






Mathematics, 29.01.2020 01:54

Social Studies, 29.01.2020 01:54

Mathematics, 29.01.2020 01:54

Biology, 29.01.2020 01:54


English, 29.01.2020 01:54

Mathematics, 29.01.2020 01:55

Chemistry, 29.01.2020 01:55


Mathematics, 29.01.2020 01:55

Biology, 29.01.2020 01:55



Mathematics, 29.01.2020 01:55


![[H^+]=\sqrt{(K_aC)}](/tpl/images/0530/6657/2da9a.png)
![pH=-\log [H^+]](/tpl/images/0530/6657/37e81.png)
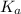 (dissociation constant) increases then the value of hydrogen ion concentration also increases and the value of pH decreases.
(dissociation constant) increases then the value of hydrogen ion concentration also increases and the value of pH decreases.

