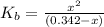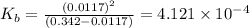Chemistry, 02.03.2020 20:38 mrmendrala
N the laboratory, a general chemistry student measured the pH of a 0.342 M aqueous solution of ethylamine, C2H5NH2 to be 12.067. Use the information she obtained to determine the Kb for this base.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
N the laboratory, a general chemistry student measured the pH of a 0.342 M aqueous solution of ethyl...
Questions

Mathematics, 12.07.2019 23:00

History, 12.07.2019 23:00

Mathematics, 12.07.2019 23:00



Mathematics, 12.07.2019 23:00


Mathematics, 12.07.2019 23:00

Mathematics, 12.07.2019 23:00



Mathematics, 12.07.2019 23:00


Mathematics, 12.07.2019 23:00



Mathematics, 12.07.2019 23:00

Mathematics, 12.07.2019 23:00

Social Studies, 12.07.2019 23:00

Mathematics, 12.07.2019 23:00

 of the an ethylamine is
of the an ethylamine is  .
.![pOH=-\log[OH^-]](/tpl/images/0530/5572/fe336.png)
![1.933=-\log[OH^-]](/tpl/images/0530/5572/b301a.png)
![[OH^-]=0.0117 M](/tpl/images/0530/5572/660e1.png)
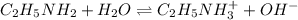
![K_b=\frac{[C_2H_5NH_3^{+}][OH^-]}{[C_2H_5NH_2]}](/tpl/images/0530/5572/63c9d.png)