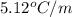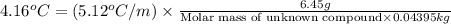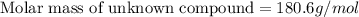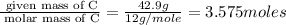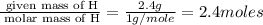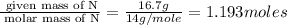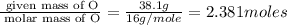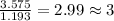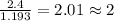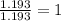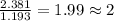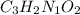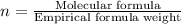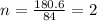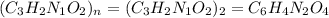Chemistry, 02.03.2020 20:25 EliHarris517
A compound is 42.9% C, 2.4% H, 16.7% N, and 38.1% O, by mass. Addition of 6.45 g of this compound to 50.0 mL benzene, lowers the freezing point from 5.53 to What is the molecular formula of this compound?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
A compound is 42.9% C, 2.4% H, 16.7% N, and 38.1% O, by mass. Addition of 6.45 g of this compound to...
Questions



Advanced Placement (AP), 09.09.2021 18:00



Spanish, 09.09.2021 18:00


English, 09.09.2021 18:00

Mathematics, 09.09.2021 18:00


Mathematics, 09.09.2021 18:00


History, 09.09.2021 18:00

Mathematics, 09.09.2021 18:00

Computers and Technology, 09.09.2021 18:00

Social Studies, 09.09.2021 18:00

Mathematics, 09.09.2021 18:00

Social Studies, 09.09.2021 18:10


English, 09.09.2021 18:10

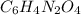
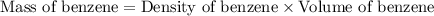
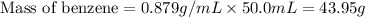
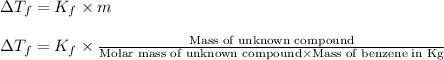
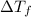 = change in freezing point =
= change in freezing point = 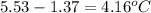
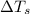 = freezing point of solution
= freezing point of solution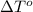 = freezing point of benzene
= freezing point of benzene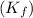 for benzene =
for benzene =