

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1

Chemistry, 23.06.2019 09:30
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2
You know the right answer?
The combustion of propane (C 3H 8) produces CO 2 and H 2O: C 3H 8 (g) 5O2 (g) 3CO2 (g) 4 H 2O (g) Th...
Questions


SAT, 03.08.2019 20:30

Mathematics, 03.08.2019 20:30



Advanced Placement (AP), 03.08.2019 20:30


Mathematics, 03.08.2019 20:30

SAT, 03.08.2019 20:30


History, 03.08.2019 20:30

Biology, 03.08.2019 20:30


History, 03.08.2019 20:30




English, 03.08.2019 20:30

Biology, 03.08.2019 20:30

English, 03.08.2019 20:30

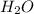 produced will be, 2 moles
produced will be, 2 moles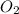 = 2.5 mol
= 2.5 mol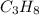 = 4.6 mol
= 4.6 mol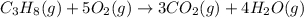
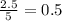 moles of
moles of 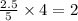 moles of
moles of 

