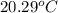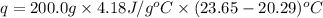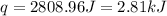A 100.0 mL of 0.500 M HBr at 20.29 oC is added to 100.0 mL of 0.500 M KOH (also at 20.29 oC). After mixing, the temperature rises to 23.65 oC. Calculate the heat of this reaction. [assuming the density and specific heat of HBr and KOH is the same as water, 1.0 g/mL; 4.18 J/g oC, and the volume of the solution is additive].

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1


Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
A 100.0 mL of 0.500 M HBr at 20.29 oC is added to 100.0 mL of 0.500 M KOH (also at 20.29 oC). After...
Questions

Social Studies, 02.12.2020 20:40


Mathematics, 02.12.2020 20:40

Arts, 02.12.2020 20:40



Geography, 02.12.2020 20:40


Physics, 02.12.2020 20:40


Mathematics, 02.12.2020 20:40



Mathematics, 02.12.2020 20:40

English, 02.12.2020 20:40


Mathematics, 02.12.2020 20:40

Mathematics, 02.12.2020 20:40


Biology, 02.12.2020 20:40

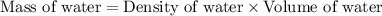
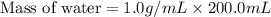
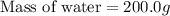
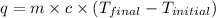
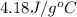
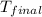 = final temperature =
= final temperature = 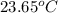
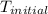 = initial temperature =
= initial temperature =