
Chemistry, 02.03.2020 19:22 PONBallfordM89
A 11.6 g piece of metal is heated to 98°C and dropped into a calorimeter containing 50.0 g of water (specific heat capacity of water is 4.18 J/g°C) initially at 20.5°C. The empty calorimeter has a heat capacity of 125 J/K. The final temperature of the water is 28.2°C. Ignoring significant figures, calculate the specific heat of the metal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

You know the right answer?
A 11.6 g piece of metal is heated to 98°C and dropped into a calorimeter containing 50.0 g of water...
Questions


Chemistry, 26.07.2019 09:00





Mathematics, 26.07.2019 09:00


Chemistry, 26.07.2019 09:00

Mathematics, 26.07.2019 09:00


Mathematics, 26.07.2019 09:00

Biology, 26.07.2019 09:00


English, 26.07.2019 09:00


History, 26.07.2019 09:00




![q=-[q_1+q_2]](/tpl/images/0530/4537/f9283.png)
![m\times c\times (T_f-T_1)=-[c_1\times (T_f-T_2)+m_2\times c_2\times (T_f-T_2)]](/tpl/images/0530/4537/372b8.png)
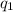 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter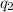 = heat absorbed by the water
= heat absorbed by the water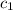 = specific heat of calorimeter =
= specific heat of calorimeter = 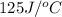
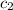 = specific heat of water =
= specific heat of water = 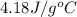
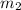 = mass of water = 50.0 g
= mass of water = 50.0 g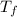 = final temperature =
= final temperature = 
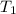 = temperature of metal =
= temperature of metal = 
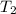 = temperature of water =
= temperature of water = 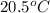
![11.6g\times c\times (28.2-98)^oC=-[125J/^oC\times (28.2-20.5)^oC+50.0g\times 4.18J/g^oC\times (28.2-20.5)^oC]](/tpl/images/0530/4537/17b20.png)



