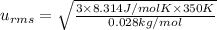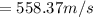Chemistry, 02.03.2020 19:29 firenation18
A 0.50 m3 gas tank holds 3.0 moles of ideal diatomic nitrogen gas at a temperature of 350 K. The atomic mass of nitrogen is 14 g/mol. What is the rms speed of the molecules? (The Boltzmann constant is 1.38 × 10-23 J/K, NA = 6.022 × 1023 molecules/mol.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

You know the right answer?
A 0.50 m3 gas tank holds 3.0 moles of ideal diatomic nitrogen gas at a temperature of 350 K. The ato...
Questions

Mathematics, 27.01.2020 18:31


Mathematics, 27.01.2020 18:31


Mathematics, 27.01.2020 18:31


Biology, 27.01.2020 18:31

Biology, 27.01.2020 18:31



Mathematics, 27.01.2020 18:31


Mathematics, 27.01.2020 18:31


World Languages, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31


History, 27.01.2020 18:31

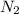 , M= 2 × 14 g/mol = 28 g/mol
, M= 2 × 14 g/mol = 28 g/mol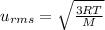
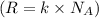
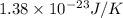
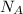 = Avogadro number
= Avogadro number