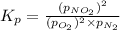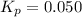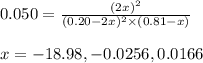Chemistry, 02.03.2020 19:27 BobBball9126
In a chamber filled with air (look up the partial pressures of nitrogen and oxygen gases) at 2200 °C, the reaction that generates nitrogen monoxide from nitrogen gas and oxygen gas has a Kp = 0.050. What are the equilibrium partial pressures of the three gases in the above reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
In a chamber filled with air (look up the partial pressures of nitrogen and oxygen gases) at 2200 °C...
Questions


Mathematics, 26.01.2022 19:30

Mathematics, 26.01.2022 19:30






Physics, 26.01.2022 19:30




English, 26.01.2022 19:30



Mathematics, 26.01.2022 19:40

Mathematics, 26.01.2022 19:40

Mathematics, 26.01.2022 19:40

Mathematics, 26.01.2022 19:40

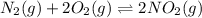
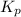 for above equation follows:
for above equation follows: