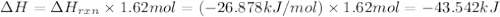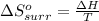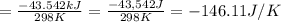Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
I2(g) + Cl2(g)2ICl(g) Using standard thermodynamic data at 298K, calculate the entropy change for th...
Questions




Mathematics, 22.04.2020 01:12







Social Studies, 22.04.2020 01:12






Mathematics, 22.04.2020 01:12

Mathematics, 22.04.2020 01:12


History, 22.04.2020 01:12

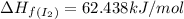
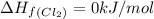
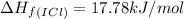
![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0530/3455/db29b.png)
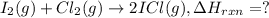
![\Delta H_{rxn}=[(2\times \Delta H_f_{(ICl)})]-[(1\times \Delta H_f_{(I_2)})+(1\times \Delta H_f_{(Cl_2)})]](/tpl/images/0530/3455/4a661.png)
![=[2\times 17.78 kJ/mol]-[1\times 0 kJ/mol+1\times 62.436 kJ/mol]=-26.878 kJ/mol](/tpl/images/0530/3455/6a4a8.png)