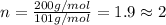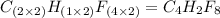Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2

Chemistry, 23.06.2019 10:00
Lord kelvin described the concept of absolute zero temperature and the laws relating to the change of thermal energy during chemical reactions what type of chemist would he be considered today
Answers: 1
You know the right answer?
A compound with the empirical formula C2HF4 was found to have a molar mass of about 200g/mole. What...
Questions

Mathematics, 11.03.2021 22:00


Mathematics, 11.03.2021 22:00

Mathematics, 11.03.2021 22:00

History, 11.03.2021 22:00

English, 11.03.2021 22:00

Health, 11.03.2021 22:00

Chemistry, 11.03.2021 22:00




History, 11.03.2021 22:00

English, 11.03.2021 22:00

Mathematics, 11.03.2021 22:00

English, 11.03.2021 22:00




Mathematics, 11.03.2021 22:00

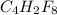 is the molecular formula of the compound.
is the molecular formula of the compound.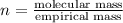
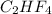 :
: