

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Write the chemical equations involved in this experiment and how that the rate of disappearance of [...
Questions

Mathematics, 18.04.2020 03:49


Mathematics, 18.04.2020 03:49


Mathematics, 18.04.2020 03:50



Mathematics, 18.04.2020 03:50






Mathematics, 18.04.2020 03:50



Mathematics, 18.04.2020 03:50


Mathematics, 18.04.2020 03:50

Biology, 18.04.2020 03:50

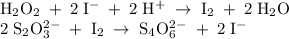
![rate = \frac{dI2}{dt} = \frac{1/2 [S2O3^2^-]}{t}](/tpl/images/0530/1057/693ef.png)


