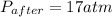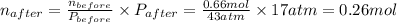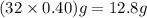A rigid tank contains 0.66 mol of oxygen (O2). Find the mass of oxygen that must be withdrawn from the tank to lower the pressure of the gas from 43 atm to 17 atm. Assume that the volume of the tank and the temperature of the oxygen are constant during this operation. Answer in units of g.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
You know the right answer?
A rigid tank contains 0.66 mol of oxygen (O2). Find the mass of oxygen that must be withdrawn from t...
Questions

Chemistry, 03.07.2019 03:00


History, 03.07.2019 03:00


Geography, 03.07.2019 03:00

Mathematics, 03.07.2019 03:00


Mathematics, 03.07.2019 03:00

Mathematics, 03.07.2019 03:00




Mathematics, 03.07.2019 03:00


Mathematics, 03.07.2019 03:00


Mathematics, 03.07.2019 03:00


Computers and Technology, 03.07.2019 03:00

Mathematics, 03.07.2019 03:00

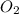 must be withdrawn from tank
must be withdrawn from tank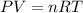
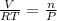
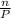 ratio will also be constant before and after removal of
ratio will also be constant before and after removal of 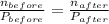
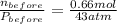 and
and