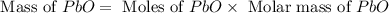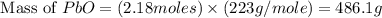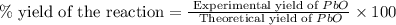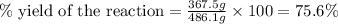Chemistry, 02.03.2020 16:32 StupidFatChipmunk
Consider the reaction. 2 Pb ( s ) + O 2 ( g ) ⟶ 2 PbO ( s ) An excess of oxygen reacts with 451.4 g of lead, forming 367.5 g of lead(II) oxide. Calculate the percent yield of the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Consider the reaction. 2 Pb ( s ) + O 2 ( g ) ⟶ 2 PbO ( s ) An excess of oxygen reacts with 451.4 g...
Questions

Physics, 11.07.2019 14:00




Mathematics, 11.07.2019 14:00

Mathematics, 11.07.2019 14:00

Physics, 11.07.2019 14:00

Health, 11.07.2019 14:00

Mathematics, 11.07.2019 14:00

Mathematics, 11.07.2019 14:00

History, 11.07.2019 14:00

History, 11.07.2019 14:00

History, 11.07.2019 14:00


Mathematics, 11.07.2019 14:00




History, 11.07.2019 14:00

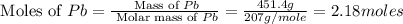
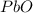
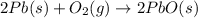
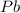 react to give 2 mole of
react to give 2 mole of