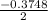Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1


Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
Calculate the number of moles of Cl2 produced at equilibrium when 3.98 mol of PCl5 is heated at 283....
Questions

Business, 20.10.2021 03:10






Mathematics, 20.10.2021 03:10

English, 20.10.2021 03:10



Physics, 20.10.2021 03:10

Social Studies, 20.10.2021 03:10

History, 20.10.2021 03:10

Mathematics, 20.10.2021 03:10

Mathematics, 20.10.2021 03:10


Mathematics, 20.10.2021 03:10



History, 20.10.2021 03:10

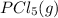 ⇄
⇄ 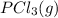 +
+ 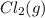
![\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0529/9897/247b7.png)
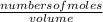
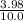
![\frac{[x][x]}{[0.398-x]}](/tpl/images/0529/9897/2afb6.png)
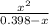
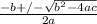
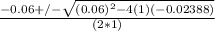
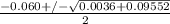
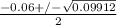
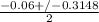
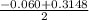 or
or 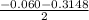
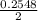 or
or