

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1
You know the right answer?
A molecule has the empirical formula C4H6O. If its molecular weight is determined to be about 212 g/...
Questions

History, 13.01.2020 19:31

Mathematics, 13.01.2020 19:31

English, 13.01.2020 19:31

Physics, 13.01.2020 19:31

Mathematics, 13.01.2020 19:31

Mathematics, 13.01.2020 19:31



Mathematics, 13.01.2020 19:31

Mathematics, 13.01.2020 19:31


Physics, 13.01.2020 19:31


Mathematics, 13.01.2020 19:31


English, 13.01.2020 19:31



English, 13.01.2020 19:31

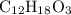 .
. 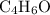 , then the molecular formula would be
, then the molecular formula would be 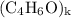 , or equivalently
, or equivalently 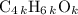 , where
, where  is a positive whole number (
is a positive whole number (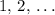 , etc.) The goal here is to find the value of
, etc.) The goal here is to find the value of 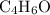 would be
would be .
.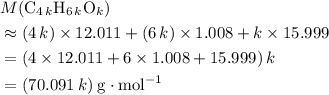 .
. ,
, 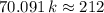 .
. . (Round to the nearest whole number.)
. (Round to the nearest whole number.)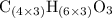 , which simplifies to
, which simplifies to 


