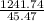If a piece of aluminum with a mass of 3.99 g and a temperature of 100.0 °C is dropped
in 10.0...

Chemistry, 29.02.2020 23:23 KaitlynLucas5132
If a piece of aluminum with a mass of 3.99 g and a temperature of 100.0 °C is dropped
in 10.0 cm3 of water at 21.0 °C, what will be the final temperature of the system?
(Hint: Remember the density of water is 1.00 g / mL)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
Questions


Mathematics, 20.10.2021 21:10


French, 20.10.2021 21:10

Mathematics, 20.10.2021 21:10



Mathematics, 20.10.2021 21:10

History, 20.10.2021 21:10


Social Studies, 20.10.2021 21:20


Social Studies, 20.10.2021 21:20


Computers and Technology, 20.10.2021 21:20

Social Studies, 20.10.2021 21:20

Mathematics, 20.10.2021 21:20

History, 20.10.2021 21:20