
Chemistry, 29.02.2020 04:26 sierra6816
A flask is filled with 6.0 atm of N2 and 6.0 atm of H2. The gases react and NH3 is formed. What is the pressure in the flask after the reaction occurs as completely as possible

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
A flask is filled with 6.0 atm of N2 and 6.0 atm of H2. The gases react and NH3 is formed. What is t...
Questions

Mathematics, 20.09.2020 14:01

History, 20.09.2020 14:01



Geography, 20.09.2020 14:01

Advanced Placement (AP), 20.09.2020 14:01






Computers and Technology, 20.09.2020 14:01


Mathematics, 20.09.2020 14:01

English, 20.09.2020 14:01


Computers and Technology, 20.09.2020 14:01



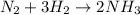
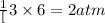 of nitrogen gas
of nitrogen gas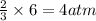 of ammonia
of ammonia


